
Afshin Beheshti, PhD
Associate Investigator
Center of Cancer Systems Biology
Research Assistant Professor of Medicine
Tufts University School of Medicine
Email: afshin.beheshti [at] tufts.edu or afshin [at] cancer-systems-biology.org
Education and Training:
| 2000 – 2002 | PhD, Biophysics | Florida State University, Tallahassee, FL |
| 1997 – 2000 | MS, Physics | Florida State University, Tallahassee, FL |
| 1993 – 1997 | BS, Physics | University of Minnesota, Minneapolis, MN |
Research Interests:
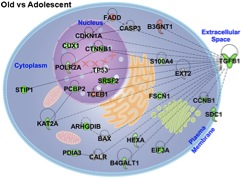
Aging Effects on Carcinogenesis
Aging has tremendous impact on cancer progression and is
important to consider when assessing the carcinogenesis process
and individualized cancer therapy. With increasing host age,
normally robust cellular processes such as angiogenesis or
immune surveillance may decrease resulting in a change in
tumor-host dynamics. My interest and research is focused on the
effects and mechanisms of how older hosts provide a
microenvironment that facilitates slower tumor
progression. Using murine tumor models at various ages along
with mathematical modeling and advanced gene
array/bioinformatics tools, I am able to start unraveling the
mystery behind the tumor dynamics with advanced age.
 |
 |
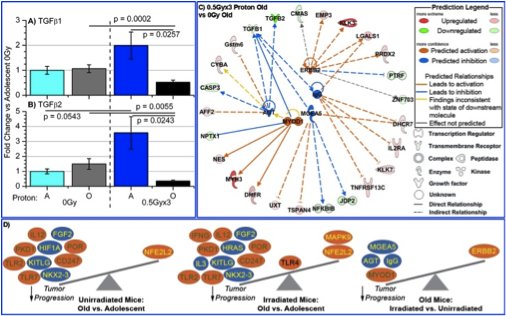
Proton and HZE Irradiation Effects with Aging on
Carcinogenesis
Proton radiation is touted for improved tumor targeting, over
standard gamma radiation, due to the physical advantages of ion
beams for radiotherapy. Recent studies from our laboratory
demonstrate that in addition to targeting advantages, proton
irradiation can inhibit angiogenic and immune factors critical to
hallmark cancer processes and thereby modulate tumor
progression. Beyond therapy, high-energy protons constitute a
principal component of galactic cosmic rays followed by other type
of HZE (high atomic number (Z), high energy (E)) radiation, such as
56Fe. These factors are a consideration in carcinogenesis
risk for space flight. Given that proton irradiation modulates
fundamental biological processes that are known to decrease with
host aging, angiogenesis and immunogenicity, I am investigating how
proton irradiation impacts tumor advancement as a function of host
age, a question with both therapeutic and carcinogenesis
implications. In addition I am also studying the effects of 56Fe
irradiation as a function of host age which is a subject that has
been minimally explored.
 |
 |
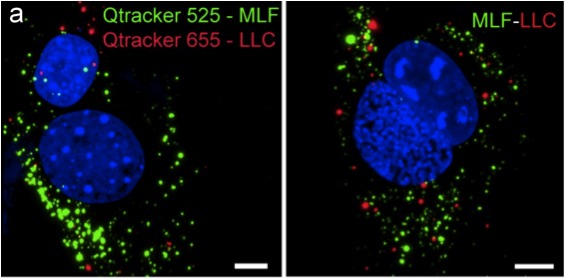
Cell fusion has been known to occur naturally in nature in
muscle formation, neurons, stem cells, and embryogenesis. With the
assistance of viruses, cell fusion has also been implicated in
tumorigenesis. I'm interested in tumor–cell fusion and other
fusion that can take place in the natural process of tumorigenesis.
DNA Double Strand Breaks (DSBs) have been widely studied
in the radiation field. DNA DSBs occur when both strands in the
double helix are severed. This is particularly hazardous to the
cell because they can lead to genome rearrangements and
mutations which can eventually lead to cancer. DNA DSBs can be
caused by many factors such as radiation, oxidation, UV light,
industrial chemicals, etc. When a DNA DSB occurs in a cell,
there are two methods of repair which can occur: non-homologous end
joining (NHEJ) and homologous recombination (HR). Both of these
methods have proteins that become phosphorylated or recruited
for the repair. Some of these repair proteins of particular
interest are γ-H2AX, 53BP1, and phosphorylated ATM
(psATM). As seen in the images below, these proteins all
colocalize and appear as foci where there are DSBs in the nuclei
of a cell. In healthy cells, there are minimal amounts of DSBs
that occur in the cell (on average of less than 1 foci per
cell), whereas cancer cells have a wide variety of DNA DSBs. My
interest is in the interaction of these cancer cells with a
naturally-elevated level of DSBs and the impact on the
microenvironment.

Publications:
(click on title to go to manuscript abstract)
- Beheshti A, Wage J, McDonald JT, Lamont C, Peluso M,
Hahnfeldt P, Hlatky L. Tumor-host signaling interaction reveals a
systemic, age-dependent splenic immune influence on tumor
development. Oncotarget. 2015 Oct 21. DOI:
10.18632/oncotarget.6214. [Epub ahead of print] [ Open Access ]
- Benzekry S, Beheshti A, Hahnfeldt P, Hlatky L. Capturing
the driving role of tumor-host crosstalk in a dynamical model of
tumor growth. Bio-Protocol. In press,
2015.
- Wage J, Ma L, Peluso M, Lamont C, Evens AM, Hahnfeldt P,
Hlatky L, Beheshti A. Proton irradiation impacts age-driven modulations
of cancer progression influenced by immune system transcriptome
modifications from splenic tissue. J Radiat Res. 2015
Sep;56(5):792-803. Epub 2015 Aug 7. PMCID: PMC4577010. [ Open
Access ]
- Suresh Kumar MA, Peluso M, Chaudhary P, Dhawan J,
Beheshti A, Manickam K, Thapar U, Pena L, Natarajan M, Hlatky L, Demple B, Naidu M. Fractionated radiation exposure of rat spinal cords leads to
latent neuro-inflammation in brain, cognitive deficits, and
alterations in Apurinic Endonuclease 1. PLoS
One. 2015 Jul 24;10(7):e0133016. PMCID: PMC4514622. [ Open Access ]
- Beheshti A, Benzekry S, McDonald JT, Ma L, Peluso M, Hahnfeldt P,
Hlatky L. Host age is a systemic regulator of gene expression impacting cancer progression. Cancer Res. 2015 Mar
15;75(6):1134-43. Epub 2015 Mar 2. PMCID: PMC4397972.
- Benzekry S, Lamont C, Beheshti A, Tracz A, Ebos JML,
Hlatky L, Hahnfeldt P. Classical mathematical models for
description and prediction of experimental tumor
growth. PLOS Comput Biol. 2014 Aug
28;10(8):e1003800. eCollection 2014. PMCID: PMC4148196. [ Open Access ]
 Beheshti A, Peluso M, Lamont C, Hahnfeldt P, Hlatky
L. Proton irradiation
augments the suppression of tumor progression observed with advanced
age. Radiat Res. 2014 Mar;181(3):272-83. Epub 2014 Feb
25. PMCID: PMC4406472. COVER
ARTICLE
Beheshti A, Peluso M, Lamont C, Hahnfeldt P, Hlatky
L. Proton irradiation
augments the suppression of tumor progression observed with advanced
age. Radiat Res. 2014 Mar;181(3):272-83. Epub 2014 Feb
25. PMCID: PMC4406472. COVER
ARTICLE- Beheshti A, Pinzer BR, McDonald JT, Stampanoni M,
Hlatky L. Early tumor development captured through
nondestructive, high resolution differential phase contrast X-ray
imaging. Radiat Res. 2013 Nov;180(5):448-54. Epub 2013 Oct
14. PMCID: PMC3925470.
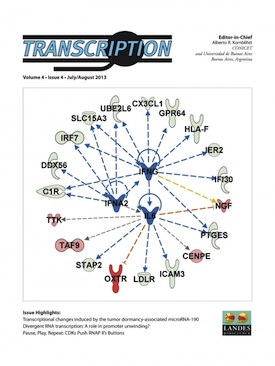 Almog N, Briggs C, Beheshti A, Ma L, Wilkie KP, Rietman E, Hlatky L. Transcriptional changes induced by the tumor
dormancy-associated microRNA-190. Transcription. 2013 Jul
1;4(4). PMCID: PMC3977918. [ Open Access ] COVER
ARTICLE
Almog N, Briggs C, Beheshti A, Ma L, Wilkie KP, Rietman E, Hlatky L. Transcriptional changes induced by the tumor
dormancy-associated microRNA-190. Transcription. 2013 Jul
1;4(4). PMCID: PMC3977918. [ Open Access ] COVER
ARTICLE
- Beheshti A, Sachs RK, Peluso M, Rietman E, Hahnfeldt P,
Hlatky L. Age and space irradiation modulate tumor progression:
implications for carcinogenesis risk. Radiat Res. 2013
Feb;179(2):208-20. Epub 2013 Jan 4.
- Almog N, Ma L, Schwager C, Brinkmann BG, Beheshti A,
Vajkoczy P, Folkman J, Hlatky L, Abdollahi A. Consensus micro RNAs governing the switch of dormant
tumors to the fast-growing angiogenic phenotype. PLoS
One. 2012;7(8):e44001. Epub 2012 Aug 31. PMCID: PMC3432069. [ Open Access ]
- Domhan S, Ma L, Tai A, Anaya Z, Beheshti A, Zeier M,
Hlatky L, Abdollahi A. Intercellular communication by exchange of
cytoplasmic material via tunneling nano-tube like structures in
primary human renal epithelial cells. PLoS
One. 2011;6(6):e21283. Epub 2011 Jun 27. PMCID: PMC3124493. [ Open Access ]
- Enderling H, Anderson AR, Chaplain MA, Beheshti A, Hlatky
L, Hahnfeldt P. Paradoxical dependencies of tumor dormancy and
progression on basic cell kinetics. Cancer Res. 2009 Nov
15;69(22):8814-21. Epub 2009 Nov 3. [ Open Access ]
- Rill RL, Beheshti A, Van Winkle DH. DNA
electrophoresis in agarose gels: effects of field and gel
concentration on the exponential dependence of reciprocal mobility
on DNA length. Electrophoresis. 2002 Aug;23(16):2710-9.
- Van Winkle DH, Beheshti A, Rill RL. DNA electrophoresis in agarose gels: a simple
relation describing the length dependence of
mobility. Electrophoresis. 2002 Jan;23(1):15-9.
- Beheshti A. DNA electrophoresis in
agarose gels: a new mobility vs. DNA length
dependence. Florida State University dissertation thesis,
2002.